Molar Mass of Cl
Examples of molecular weight computations. Enter a chemical formula.

How To Calculate The Molar Mass Of A Compound Quick Easy Youtube
The molar mass links the mass of a substance to its moles.

. C 18 H 38. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. 4 Be Beryllium 90121831.
The first molar ionization energy applies to the neutral atoms. This way we can calculate the molar mass of a compound or one-carbon compound. 1 atom x 23 gramsmole Na 1 atom x 355 gramsmole Cl 585 gramsmole NaCl.
Molar mass g mol chlorine. See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. Molar mass of a compound is sum of molar masses of all the atoms present in that compound respective of the number of atoms of each element.
Molar concentration is the amount of a solute present in one unit of a solution. 610 amu 300 amu. Molar mass of H 2 O can be calculated using the following formula.
The mass of the sample containing about 6 023 1 0 23 6023 times 10. How does Atomic Mass Calculator work. The molar mass of a substance depends not only on its molecular formula but also on the distribution of isotopes of each chemical element present in it.
Molar Concentration of Ions Problem. For example the molar mass of calcium-40 is 39962 590 98 22 gmol whereas the molar mass of calcium-42 is 41958 618 01 27 gmol and of calcium with the normal isotopic mix is 400784 gmol. Stoichiometry Percent Composition One can find the percentage of the mass of a compound.
Modification of work by the Italian voiceFlickr. C 10 H 8. First of all every element has a different molar mass and is expressed as gram per mole.
If you have a subscript in a chemical formula. Molar mass of C 2 H 3 Cl PVC should be calculated. 6 C Carbon 12011.
Atomic mass of Oxygen 1600. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. However some of these ions associate with each other in the solution leading to a decrease in the total number of particles in the solution.
Some facts The molar mass is simply the mass of one mole of substance ie. 2a Using 0002 gL caculate the molarity. Lets consider the saturated solution of silver.
2 He Helium 4002602. Thus whether you type a chemical formula for a compound like CaO or type chemical symbol for a single element like Na it will show atom count molar mass and. Find the molarity of the solute.
Modification of work. 2355 amu 1111 amu These are generally reported for ionic compounds. Definitions of molecular mass molecular weight molar mass and molar weight.
Well add those numbers together along with the unit grams per mole for finding molar mass. Molar mass g mol octadecane. If we have a chemical compound like NaCl the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine.
What is molar concentration. Divide each mole value by the small number of moles you obtained from your calculation. What This Calculator Does and Why You Should Use It.
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter its formula specify its isotope mass number after each element in square brackets. We can also use molecular weight calculator for finding molar mass of a.
If we write this as a calculation it looks like this. Atomic mass of Cu 6355 Atomic mass of Cl 3545 Atomic mass of CuCl 2 16355. The second third etc molar ionization energy applies to the further removal of an electron from a singly doubly etc charged ion.
Select elements and see the molar mass of the compound. Modification of work by vxlaFlickr. 7 N Nitrogen 14007.
Isocane eicosane C 20 H 42. It is 5844 g mol 1. Its units are molL moldm 3 or molm 3.
2120 amu H. Moreover most of the molecules are made up of more than 1 element. If we have to measure one mole of sodium.
8 O Oxygen 15999. For example the Chlorine Cl has a molar mass of 354530 gmol in the same way Sodium Na has a molar mass of 229898 gmol. Use the molar mass you get by adding up the atomic weight of the elements from the periodic table to convert the mass of each element into moles.
Atomic mass of Carbon 1201. Stoichiometry Molecular Weight MW Sum of the atomic weights of the atoms in a molecule For the molecule ethane C 2H 6 the molecular weight would be C. Are you facing one too many chemistry problems sets tonight.
1 Convert ppm to a gram-based concentration. 3 Li Lithium 694. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by.
The total should be 100 percent. 60663 Calculate the. 10 Ne Neon 201797.
The protons neutrons electrons calculator is developed to store the complete data of the atomic mass and subatomic masses of elements present in the periodic table. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. Let me make it more clear with an example of sodium chloride.
If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. Molar mass of Carbon Monoxide 2801. Calculate the molarity of a dye concentration given the molar mass is of the dye 327 gmol and a dye concentration of 2 ppm.
In other words set the mass of each element equal to the percent. 2764 Calculate the molecular weight of a chemical compound. M H 2 O 2 M H M O Here M H and M O is.
To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride. For example the Vant Hoff factor of CaCl 2 is ideally 3 since it dissociates into one Ca 2 ion and two Cl ions. 9 F Fluorine 18998403163.
In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units. The molar mass of sodium chloride is known. Heres an example to better demonstrate the concept.
The molar mass of the molecule is. This factor is named after the Dutch physical chemist Jacobus Henricus Vant Hoff who won the first Nobel Prize in chemistry. These tables list values of molar ionization energies measured in kJmol 1This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions.
Chemical formula Hill notation Molar mass gmol Modify Clear mnM. More information on molar mass and molecular weight. 2 ppm 2 mg dye L of solution.
Browse the list of common chemical compounds. A solution is. So for finding out the molar mass of the molecule you have.
From the periodic table. What is the molarity of the Cl ions in the solution. 0002 gL divided by 327 gmol 61 x 10-6 M.
5 B Boron 1081. Molar concentration also known as molarity and can be denoted by the unit M molar.
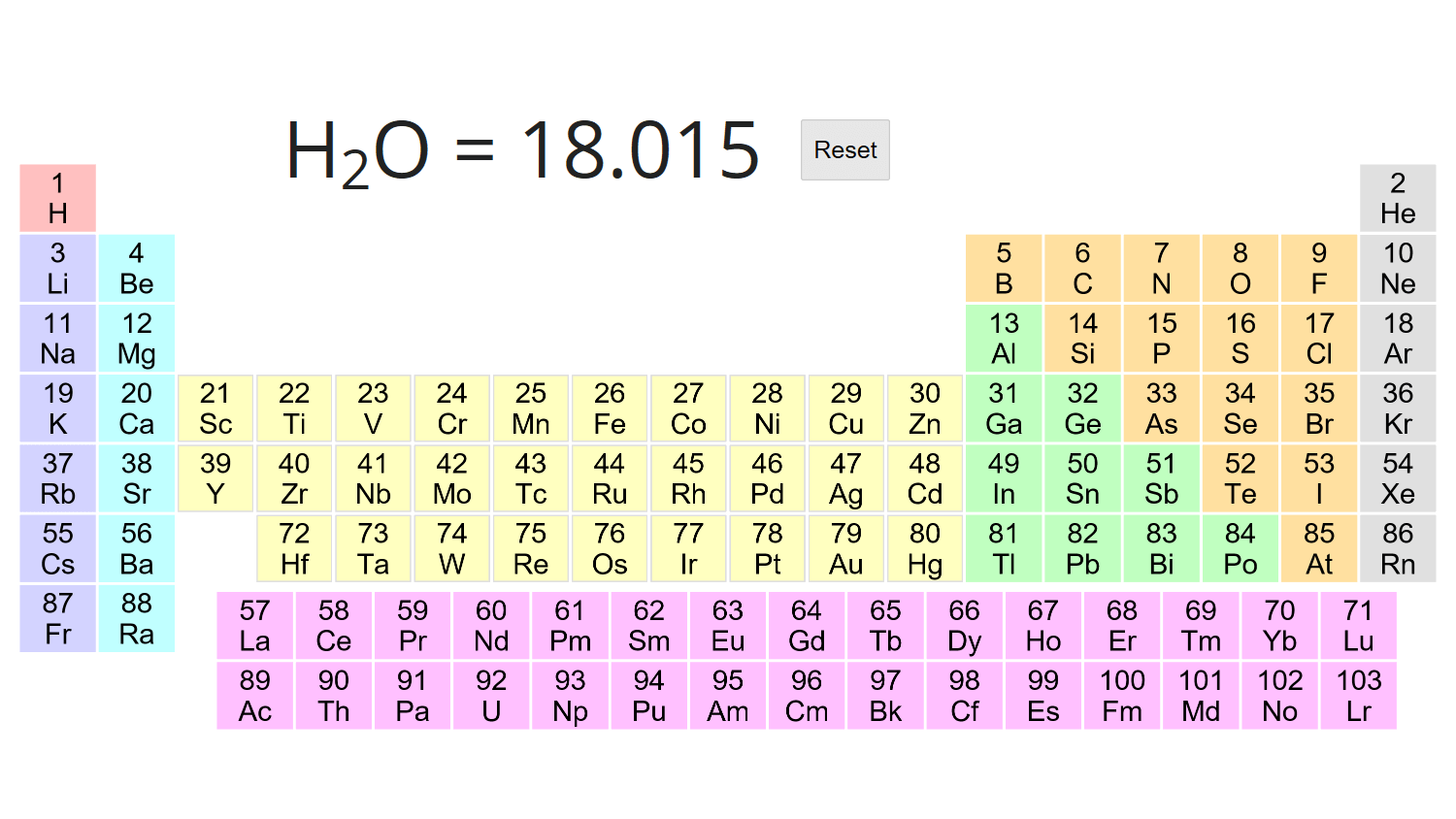
Molecular Weight Calculator Javalab

Molar Mass Molecular Weight Of Cacl2 Youtube
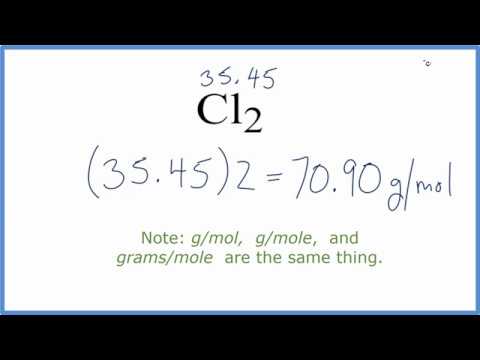
Molar Mass Molecular Weight Of Cl2 Chlorine Gas Youtube

Molar Mass Molecular Weight Of Cl2 Chlorine Gas Youtube
0 Response to "Molar Mass of Cl"
Post a Comment